Lead and tin are two metals widely used in various industries and are components of various alloys, including solders, batteries, and anti-corrosive coatings. Because these two metals are often mixed in many industrial processes, it is sometimes necessary to separate tin (Sn) and lead (Pb) for later use in high-purity products.
The process for separating tin and lead depends on the physical form of the mixture, the ratio of its components, and the final purpose (refining or extraction). Various methods can be used, such as thermal, chemical, and electrical separation. A detailed description of these methods is provided below.
1. Composition and properties of metallic lead and tin.
Lead (Pb) is a soft, heavy metal with a relatively low melting point (327 °C). Tin (Sn) is a lustrous, silvery metal with a melting point of 232 °C. The difference in the melting points of these two metals is fundamental for physical separation processes. While tin tends to form a stable oxide, lead retains its metallic form at high temperatures or decomposes into its oxide (PbO) under certain conditions. These differences underlie thermal and chemical separation processes.
2. Basic methods for separating tin and lead
a) Thermal process (selective melting)
One of the most common methods for separating tin and lead is by using melting point differences .
This involves heating the alloy to a temperature between 232 and 327 degrees Celsius. At this temperature, the tin begins to melt, while the lead remains in a semi-solid state. Partial melting can cause the tin to detach from the surface or penetrate into the solid.
Stages of implementation of the thermal method:
-
The can is gradually heated to a temperature of approximately 240–250 degrees Celsius.
-
Maintain the temperature for a while until the can is completely melted.
-
Separation of the liquid phase (molten tin) from the solid phase (lead).
-
If necessary, tin is finally cleaned chemically or electrolytically to achieve a high degree of purity.
This process is suitable for lead-rich alloys and is widely used in the solder recycling industry.
b) Selective oxidation
This process takes advantage of the different stability of tin and lead oxides. Tin readily converts to tin oxide (SnO₂) at high temperatures, while lead typically converts to PbO or PbO₂ under the same conditions. Both metals have different physical properties.
To separate them, the metal mixture is first exposed to hot air or a controlled amount of oxygen to convert the tin to SnO₂. This oxide is then separated from the lead using chemical methods such as carbon reduction or acid reactions.
General steps:
-
The mixture is heated to a temperature of 500–700 °C in the presence of oxygen.
-
Tin oxide forms on the surface.
-
Selective purification and dissolution of oxides in acid or base (e.g. hydrochloric acid to dissolve PbO and obtain SnO₂).
-
Tin is obtained from oxides by reduction processes.
This process is mainly used in the metallurgy industry to extract tin from lead solder.

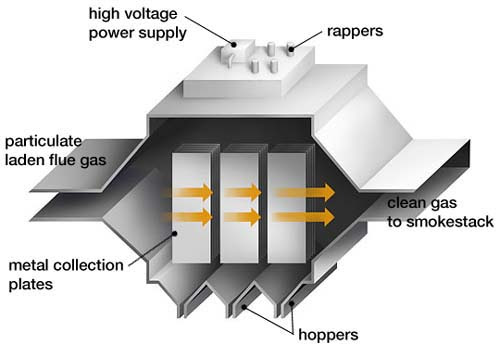
c) Electrolysis (electrorefining)
Electrolysis is one of the most precise and effective methods for metal separation.
It involves placing an alloy of lead and tin into an electrolytic cell, which serves as the anode. By adjusting the electrolyte and voltage, one metal (usually tin) dissolves , while the other (lead) deposits at the anode.
Example of electrolyte:
-
A dilute sulfuric acid solution with added chloride ions.
-
Or an alkaline solution containing tin ions in complex form.
In this process, tin dissolves in the solution as Sn²⁺ ions, which are then deposited as pure metal on the cathode by varying the electric field strength. The main advantage of this method is the exceptionally high purity of the recovered metal
and the precise control of its chemical composition. However, due to the high equipment costs and energy consumption, small-scale production is unprofitable.
d) Chemical solution method (selective extraction)
In some applications, particularly in the recycling of printed circuit boards, a dissolution process is used
in which crushed or powdered alloys are placed in a special chemical solution that dissolves one of the metals while the other remains in a solid state.
For example:
-
With nitric acid (HNO₃), tin can be dissolved in solution to form Sn(NO₃)₂ and lead can be converted and precipitated into PbSO₄ or PbO.
-
Alternatively, under certain conditions , hydrochloric acid (HCl) can be used to dissolve lead as PbCl₂.
After melting, pure tin or lead can be obtained by precipitation, chemical reduction, or electrolysis.
This process is often used for recycling electronic solders and industrial waste due to its easy control and low environmental impact.
3. Industrial application of separation processes
-
PCB recycling industry: Extraction of tin from old solder and separation of lead for reuse.
-
Industrial lead refining: The removal of tin is essential for the production of pure soft lead for lead-acid batteries.
-
Production of pure tin for food packaging: Electrolysis separates tin from lead and purifies it for use in the canning industry.
-
Soldering plant: Extraction of tin from scrap and adjustment of the solder composition, without lead contamination.
4. Safety and environmental protection tips
Both metals, especially lead, are highly toxic. Their vapors and soluble compounds can damage the nervous system and kidneys. Therefore, the following precautions should be observed when separating them :
-
Ensure adequate ventilation during heat treatment and melting.
-
Wear a mask, gloves and chemical-resistant clothing.
-
Collection and treatment of wastewater and acidic solutions.
-
Recycle waste containing heavy metals instead of disposing of it directly into the environment.
Compliance with environmental standards ensures the economic sustainability of the company and the protection of human health.
5. Conclusion
The separation of tin and lead is a fundamental process in metallurgy and mineral extraction. The choice of the appropriate method depends on the alloy type, its composition, purification objectives, and technical feasibility.
Thermal processes are suitable for simple and rapid separation, while electrolytic and chemical processes ensure separation with a high degree of purity. In addition to technical aspects,
safety and environmental protection must be prioritized to ensure a cost-effective, sustainable, and environmentally friendly process.
Recommended sources for further reading
-
ASM Handbook – Volume 15: Electronic Materials and Processes.
-
Metallurgical Processes (Haver and Lee): Chapter on smelting and refining of metals.
-
Environmental Protection Agency (EPA): Guide to Recycling Lead and Tin